A technology brief on ammonia as a fuel for maritime transport
Ammonia (NH3) is a colorless, pungent, and toxic gas. Under ambient conditions its density is lower than air and it has a boiling point of -33.3°C, but at a moderate pressure of 8.6 bar (125 psi) it can be handled as a liquid at room temperature. Ammonia has been carried as a cargo on maritime vessels for many years, primarily as a fertilizer for agriculture, but only recently has marine ammonia fuel attracted mainstream interest. It’s energy density is about half of petroleum fuels like heavy fuel oil and diesel. NH3 is often referred to as a hydrogen-carrier, since pure hydrogen can be obtained by cracking ammonia into its constituent elements.



Marine ammonia can be used in fuel cells or internal combustion engines. Since ammonia does not contain carbon, it emits no CO2 when burned or reacted. However the exhaust from an internal combustion engine and some fuel cells using NH3 can contain nitrogen oxides (NOx), a harmful pollutant. Internal combustion engine exhaust can also contain unburned fuel and nitrous oxide (N2O), the later of which is a greenhouse gas. These undesirable emissions must be addressed through exhaust gas treatment or combustion tuning. When ammonia is reacted in a fuel cell (see the ShipFC project) the resultant gases produced are primarily nitrogen, oxygen, and water vapor, though NOx is also possible for some fuel cells.
When a ship switched from petroleum-based fossil fuel to marine ammonia it would lead to nearly complete reduction in operating emissions. However, much depends on how the marine ammonia is produced, the feedstocks and processes used could have significant upstream emissions. In current engine designs, marine ammonia is likely to need a small amount of conventional pilot fuel for proper combustion. Naturally, this pilot fuel will result in emissions, but these emissions can be mitigated by using a biofuel.
Major engine manufacturers such as Caterpillar, Wartsila, Japan Engine Corporation, and MAN have already begun developing and testing internal combustion engines using ammonia as a fuel, with ships planning to be operating in the mid to late 2020s.
Global production of ammonia in 2018 was roughly 170 million metric tons, the vast majority being produced from natural gas eedstock. Global fuel consumption from ships is approximately 330 million metric tons of petroleum fuels. On an energy basis, 330 million metrics tons of petroleum fuel would equate to approximately 700 million metric tons of ammonia on an energy basis and require more than 6,500 TWh of electricity for production each year. The production volume increases required to see widespread adoption of ammonia as a fuel in the maritime industry are substantial
Each year there approximately 180 million metric tons of ammonia is produced globally. Ammonia can be produced through different processes, but the most common by far is the Haber Bosch process. The Haber-Bosch process was developed in the early 20th century to produce ammonia fertilizers. It is considered by some of be one of the most important industrial processes ever developed due to its impact on the agricultural and food productivity. Today it is the most common method of ammonia production.
The process involves passing nitrogen and hydrogen gases over an iron-based catalyst in a reaction chamber at a high temperature and pressure of approximately 400°C and 150 bar (2175 psi). The process is highly efficient, typically 90% or higher.
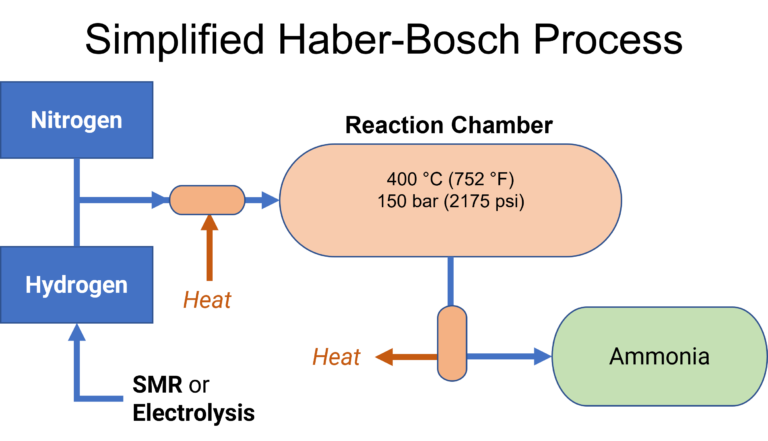
NH3 is a compound consisting of the elements nitrogen and hydrogen. These feedstocks are obtained through their own industrial processes. Hydrogen (also a marine fuel) is often sourced from steam methane reforming (SMR), but could also be obtained from water electrolysis.
While the Haber-Bosch process is efficient, it is all also energy intensive due to the high pressures and temperatures involved. This energy intensive process results in greenhouse gas emissions, which are estimated at surpassing 2.16 tons CO2 per ton of NH3 produced given current production methods. At a global level, this suggests 389 million metric tons of CO2 each year, which doesn’t include the emissions associated with the production of hydrogen feedstocks. To make this process less carbon-intensive would require using electricity produced from renewable energy for both the Haber-Bosch process and water electrolysis for hydrogen.
Ammonia can be produced through many different pathways using different feedstocks such as natural gas, coal, biomass and water through a variety of conversion steps such as steam methane reforming, gasification, or electrolysis that all rely on energy inputs. If the hydrogen is sourced from electrolysis (a very energy intensive process) powered by clean electricity, the resultant ammonia is often referred to as e-ammonia (where ‘e’ indicates electricity was used in production), one of several electrofuels or e-fuels. Currently most ammonia is produced using steam methane reforming (SMR) of natural gas to obtain the hydrogen, this is referred to as brown ammonia. Sometimes SMR is integrated with carbon capture and storage to prevent CO2 emissions and during production, this is referred to as blue ammonia
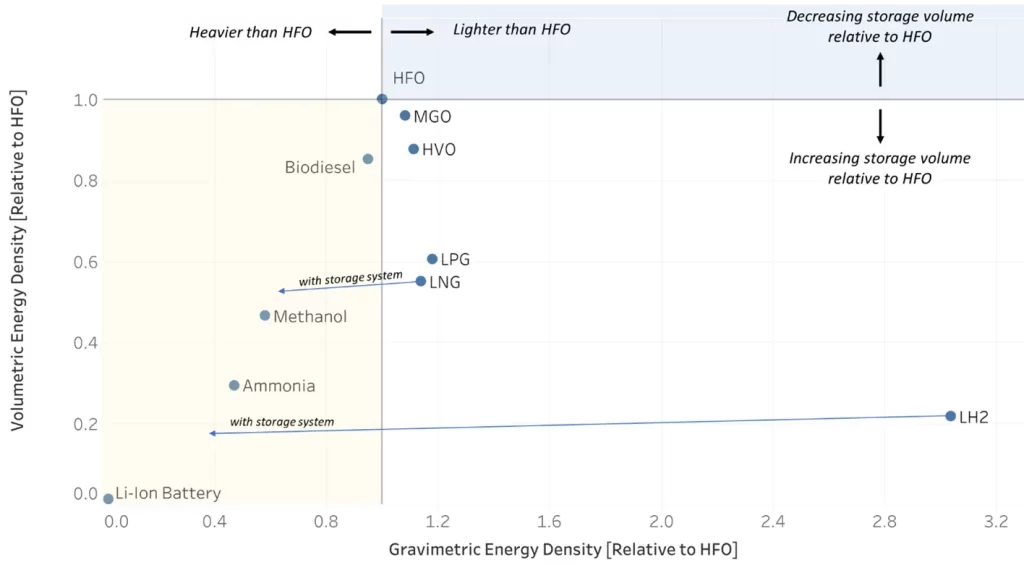
As shown in the chart below, ammonia has an energy content that is less than half on a mass basis and around 30% on a volume basis when compared to heavy fuel oil, the current standard for maritime fuels. These values do not include the storage systems needed for safe handling of ammonia which reduces its effective energy density. Aboard a vessel, this energy density challenge translates into larger volumes of fuel that need to be stored on the vessel, and more weight, for a given value of energy required and all else being equal.
Ammonia is 17.6% hydrogen by mass, and is sometimes considered a method of hydrogen storage, which has benefits over storage of hydrogen itself. Whereas hydrogen has to be stored at -253 ºC as a liquid, or at pressures of around 700 bar as a gas, liquid ammonia can be stored at a more reasonable temperature of -33 ºC at ambient pressure, and 20 ºC at 9 bar of pressure. That makes storing and transporting ammonia easier and less expensive than hydrogen given current technologies.
When storing and transporting large volumes of ammonia, it is either a pressurized liquid at 9 to 10 bar at room temperature) or a refrigerated liquid at temperatures below –33 °C and ambient pressure. Ammonia has been carried as a cargo aboard vessels for decades. These ships can carry upwards of 80,000 cubic meters of ammonia at a time. Given the large volume of cargo tanks of a ship, and therefore the large amount of surface area, ammonia is commonly stored as a refrigerated liquid aboard ships to keep the pressure low. These ammonia tanker vessels are thought to be likely early adopters of marine ammonia as a fuel given their familiarity with safe handling, and the opportunity to use marginal amounts of their cargo as a fuel as LNG carriers commonly do.
Marine ammonia can be used as fuel in a ship’s internal combustion engine or a fuel cell to convert chemical energy into useful mechanical or electrical energy to turn a shaft or charge a battery, respectively. When ammonia is used in a fuel cell, it can be used in one of two ways. Ammonia can be used directly as a fuel in its pure form, often referred to as a direct ammonia fuel cell (DAFC), or it can be decomposed into hydrogen using a cracking reactor and the hydrogen is then fed into the fuel cell. Some of the different technologies that use ammonia for maritime applications are discussed below.
Many types of fuel cells exist, but they all follow a similar principle of operation. They consist of an electrolyte sandwiched between two plates that serve as an anode and a cathode. The electrolyte allows hydrogen ions to move between the two sides of the fuel cell by crossing a membrane that separates them. Two separate reactions take place at the anode and cathode. At the anode, a catalyst causes the hydrogen fuel to oxidize which produces protons and electrons. The protons are allowed to pass through the fuel cell membrane to the cathode. The electrons also travel to the cathode, but do so via an external circuit and the flow of these electrons produces electricity. At the cathode, a separate reaction takes place when air is introduced that produces water and heat. A single fuel cell produces a tiny amount of electrical potential (typically less than 1 Volt), so to increase the voltage individual fuels cells are stacked together to produce practical amounts of power on the Watts to Megawatts scale.
Fuel cells are typically characterized by the electrolyte which dictates the chemical reaction and operation temperatures. Some different types that are likely to be seen in maritime applications are described below.
Solid oxide fuel cells use a thin layer of a porous ceramic material. If its an oxygen ion-conducting electrolyte, it’s designated as a SOFC-O; if it uses proton-conducting electrolyte, it’s designated as a SOFC-H. SOFC-O’s typically have a higher power density than SOFC-H. SOFCs have the highest operating temperatures of all the various types of fuel cells, commonly between 500°C and 1,000°C. SOFCs can also be a type of direct ammonia fuel cell.
Advantages: Due to the high operating temperature, these fuel cells can directly use other hydrocarbon fuels (e.g. natural gas, methanol, biogas) by internally reforming the fuel (or thermal cracking for ammonia). This fuel flexibility can be attractive for a vessel owner. When combined with other waste heat recovery systems SOFCs can achieve electrical efficiencies 60% and higher. SOFC-O’s may have higher NOx output relative to other fuel cells.
Disadvantages: While the high operating temperature is an advantage in one respect, it also leads to accelerated degradation and corrosion of some system components, especially when using ammonia. For example, in one study performance decreased 7% after only 1500 hours using NH3, compared to 2% for a NH3 and H2 mixture. Also, higher quality (and often more expensive) heat-resistant materials are required to enable to reaction, so these fuel cells are often more expensive in comparison to PEM. SOFCs also take longer to start-up and are not as easy to cycle on and off which is not ideal for maritime applications.
Alkaline fuel cells (AFCs), as the name suggests, have an alkaline liquid as the electrolyte, commonly potassium hydroxide (KOH). They have been used in NASA space programs for decades and are relatively cheap in comarison to PEMFC. AFC typically have operating temperatures that vary between room temperature to 250˚C for hydrogen, and upwards of 450 ˚C for ammonia. They can achieve power-generating efficiencies near 70 percent, and have decent power densities. AFCs have some fuel flexibility and can tolerate direct use of ammonia, methanol and hydrogen, as examples. When ammonia is used as a fuel, it is most commonly cracked into pure hydrogen which is then fed to the fuel cell. Carbon monoxide and carbon dioxide can poison AFCs and adversely affect their performance, therefore most AFCs run on pure hydrogen and oxygen. New membranes are being investigated for AFC that would allow better performance with direct use ammonia.
Advantages: Can utilize inexpensive nonprecious catalysts which reduces capital costs. Relatively mature technology that has been in commercial use since the 1960s.
Disadvantages: AFCs are very sensitive to even small amounts of CO and CO2, such as found in ambient air, which can react with the electrolyte and ultimately degrade fuel cell performance.
A proton-exchange membrane fuel cell (PEMFC) is also known as a polymer electrolyte membrane (PEM) fuel cell. As the same suggests, these fuel cells have a water-based polymer membrane as the electrolyte that conducts protons. These fuel cells commonly used platinum on both the anodes and cathodes. PEMs can be characterized as low temperature or high-temperature. Ammonia can’t be used directly in a low temperature PEM, due to the simple fact that it is hard to break the nitrogen and hydrogen bonds in NH3 at low temperatures. Moreover, certain PEM membranes are not compatible with ammonia. However NH3 can be used as a source of hydrogen production via an ammonia cracker, but this requires additional volume and cost. When using pure hydrogen, these fuel cells are approximately 60% efficient. These are the most common fuel cells currently being used in the maritime industry, typically with pure hydrogen.
Advantages: Since a PEM fuel cell uses a solid electrolyte, this reduces corrosion issues that plague other fuel cell types. PEM fuel cells also have a relatively low temperature (below 120°C) which is safer for enclosed spaces. They also have a quick start-up time and good load following characteristics, helpful for a vessel propulsion engine that might change speeds frequently. The cost of PEM fuel cells is relatively low.
Disadvantages: PEM fuel cells typically require a very pure form of hydrogen and are sensitive to impurities in the fuel. They can’t directly use other fuels (e.g., natural gas or ammonia) without first converting it to hydrogen via a reformation process otherwise it will lead to ‘poisioning’ of the expensive platinum catalysts by carbon monoxide and sulfur. High temperature PEM fuel cells are a tad less susceptible to this. The low operating temperature also means less opportunities for waste heat recovery to be used in other ship systems.
An ammonia internal combustion engine (AICE) differs from a fuel cell in that the ammonia is explosively burned in the combustion chamber to produce mechanical energy as opposed to electrical energy during a fuel cell reaction. At present, ammonia is often blended with other fuels, upwards of 70/30, to ensure proper combustion. Some of the major engine manufacturers like MAN and Wärtsilä are designing engines that could run on 100% ammonia which may be commercially ready by 2023 and 2024. Engines can also be designed as dual-fuel where it can use ammonia and some other hydrocarbon fuel like LNG, diesel, or biofuels. This dual-engine provides more flexibility to a vessel owner operator facing an uncertain fuel landscape in the coming years.
Advantages: Internal combustion engines are well established in the maritime industry. Ships are designed to accommodate large engines, and the maritime workforce is familiar with their operation. Emissions from an engine operating on pure ammonia do not contain any carbon based pollutants. There is some overlap in the storage requirements for liquefied natural gas and ammonia, which may make LNG engine retrofits to ammonia easier.
Disadvantages: NH3 has a relatively high ignition temperature and slow flame propagation, both of which adversely impact its performance when used in compression ignition engines as commonly found on vessels. Blending ammonia with other fuels like bio-diesel can mitigate these issues and is common practice. But depending on the other fuel, this could increase GHG emissions and detracts from ammonia’s value proposition as a zero-carbon fuel. Ammonia engines can produce oxides of nitrogen known as NOx which is a harmful pollutant, these must be addressed through exhaust gas treatment such as a selective catalytic reduction system.
Executive Summary – Ammonia Technology Roadmap – Analysis. (n.d.). IEA. Retrieved October 23, 2022, from https://www.iea.org/reports/ammonia-technology-roadmap/executive-summary
Thanks for subscribing to The Liquid Grid! You’ll receive email updates when new articles are posted.