A technology brief on marine hydrogen fuel for maritime transport
Hydrogen (H2) is the most abundant chemical element in the universe. At standard conditions, it exists as a gas and is colorless, odorless, and non-toxic. Gaseous hydrogen (H2) can also be converted to a more concentrated form as liquid hydrogen (LH2), but this requires cooling it to −252.87 °C (−423.17 °F). Hydrogen is energy dense, approximately three times more energy per unit mass than diesel, but less when assessed on a volumetric basis. Marine hydrogen is a promising fuel for internal combustion engines or fuel cells, the latter being more common. While H2 is plentiful, it rarely exists in its pure form and often needs to be extracted from other compounds. It is produced through a variety of methods including steam methane reforming and water electrolysis.



When marine hydrogen fuel is reacted in a fuel cell it has zero greenhouse gas emissions, it only produces water and warm air. When H2 is combusted in an engine it emits trace amounts of CO2 and nitrogen oxides which must be mitigated through exhaust treatment. Therefore, the sustainability of the H2 largely depends on how it is produced, not how it is consumed.
The vast majority of H2 produced today is generated through a process called steam methane reforming (SMR) using fossil fuels such as natural gas and coal. Global production using these fossil fuels results in CO2 emissions of around 830 million tons per year, about 80% of the GHG emissions from all international shipping. H2 can be produced using other methods and feedstocks that don’t rely on fossil fuels however. Using renewable natural gas or biogas instead of natural gas for example. Carbon capture can also be used to further reduce the emissions associated with production via SMR. SMR and other production methods are discussed in more detail below.
Hydrogen is most commonly produced through a process known as steam methane reforming (SMR). SMR involves reacting high-temperature steam (700°C–1,000°C) with a methane source, like natural gas, and a catalyst. The reaction produces hydrogen, carbon monoxide, and a some carbon dioxide. Additional processes such as “water-gas shift reaction” and “pressure-swing adsorption” can improve the efficiency of SMR and the quality of the hydrogen produced. SMR can be combined with carbon capture and storage to reduce the carbon dioxide emissions produced during the reaction between 50 and 90%.
Electrolysis is a process where an electric current is applied to a compound to force a chemical decomposition. Electrolysis of water breaks results in hydrogen and oxygen. Hydrogen produced using this process can result in zero greenhouse gas emissions if the electricity comes from clean and renewable energy resources like solar PV, wind, wave, and nuclear. Currently only a very small amount of global hydrogen demand is met through electrolysis, though as more renewable energy is brought online the hope is that this method might displace steam methane reforming. Learn more about electrolysis here.
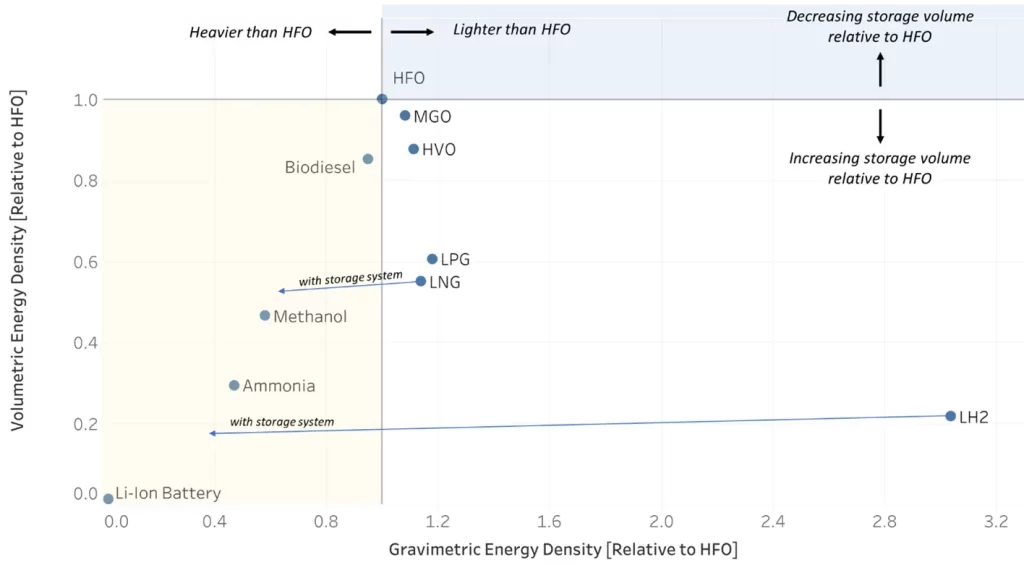
One of marine hydrogen fuel’s biggest disadvantages is how to store it cost-effectively in sufficient volumes and densities needed for maritime transport where space and weight is often a significant constraint.
Pure hydrogen can be physically stored either as a pressurized gas (CH2) at 5,000–10,000 psi, or as a cryogenic liquid near its boiling point temperatures of −252.8°C (at ambient pressure). You may remember that increasing the pressure also increases the boiling point of a liquid. Therefore cryo-compressed hydrogen (LH2), with a slightly elevated boiling point, is currently the most energy dense physical storage method. Even still, storing LH2 takes up more storage volume and weighs more relative to diesel or heavy fuel oil once the storage system is taken into account. For a given energy content a greater volume of hydrogen is required when substituting for diesel, typically 4.1 times the volume, which creates challenges for using hydrogen on large vessels. Compressed hydrogen and LH2 are both being investigated for maritime applications typically for smaller coastal or inland vessels.
Hydrogen can be stored in other ways using hydrogen carriers. Natural gas, biogas, methanol, or ammonia all contain hydrogen within their chemical structure and it can be extracted using onboard reformers or thermal cracking, but this adds additional cost, space requirements, and complexity to the overall system.
Hydrogen can be used as fuel in an internal combustion engine or a fuel cell to convert chemical energy into useful mechanical or electrical energy to turn a shaft or charge a battery, respectively.
Fuel cells are typically more efficient than internal combustion engines, especially at lower loads. They are also relatively quiet and have fewer moving parts in comparison to a diesel engine. Fuel cells require a very pure form of hydrogen however. Conversely, internal combustion engines are comparatively cheap, well-established in the maritime industry, and can accept a lower quality hydrogen.
Hydrogen fuel cells are more common than hydrogen internal combustion engines, and are discussed below in more detail. Some of these different technologies are discussed below.
Many types of fuel cells exist, but they all follow a similar principle of operation. They consist of an electrolyte sandwiched between two plates that serve as an anode and a cathode. The electrolyte allows hydrogen ions to move between the two sides of the fuel cell by crossing a membrane that separates them. Two separate reactions take place at the anode and cathode. At the anode, a catalyst causes the hydrogen fuel to oxidize which produces protons and electrons. The protons are allowed to pass through the fuel cell membrane to the cathode. The electrons also travel to the cathode, but do so via an external circuit and the flow of these electrons produces electricity. At the cathode, a separate reaction takes place when air is introduced that produces water and heat. A single fuel cell produces a tiny amount of electrical potential (typically less than 1 Volt), so to increase the voltage individual fuels cells are stacked together to produce practical amounts of power on the Watts to Megawatts scale.
Fuel cells are typically characterized by the electrolyte which dictates the chemical reaction and operation temperatures. Some different types that are likely to be seen in maritime applications are described below.
A proton-exchange membrane fuel cell (PEMFC) is also known as a polymer electrolyte membrane (PEM) fuel cell. As the same suggests, these fuel cells have a water-based polymer membrane as the electrolyte that conducts protons. These fuel cells commonly used platinum on both the anodes and cathodes. PEMs can be characterized as low temperature or high-temperature. When using pure hydrogen, these fuel cells are approximately 60% efficient. These are the most common fuel cells currently being used in the maritime industry.
Advantages: Since a PEM fuel cell used a solid electrolyte, this reduces corrosion issues that plague other fuel cell types. PEM fuel cells also have a relatively low temperature (below 120°C) which is safer for enclosed spaces. They also have a quick start-up time and good load following characteristics, helpful for a vessel propulsion engine that might change speeds frequently. The cost of PEM fuel cells is relatively low.
Disadvantages: PEM fuel cells typically require a very pure form of hydrogen and are sensitive to impurities in the fuel. They can’t directly use other hydrocarbon fuels (e.g., natural gas) without first converting it to hydrogen via a reformation process otherwise it will lead to ‘poisioning’ of the expensive platinum catalysts by carbon monoxide and sulfur. High temperature PEM fuel cells are a tad less susceptible to this. The low operating temperature also means less opportunities for waste heat recovery to be used in other ship systems.
Solid oxide fuel cells use a thin layer of a porous ceramic material. If its an oxygen ion-conducting electrolyte, it’s designated as a SOFC-O; if it uses proton-conducting electrolyte, it’s designated as a SOFC-H. SOFCs have the highest operating temperatures of all the various types of fuel cells, commonly between 500°C and 1,000°C.
Advantages: Due to the high operating temperature, these fuel cells can directly use other hydrocarbon fuels (e.g. natural gas, methanol, biogas) by internally reforming the fuel (or thermal cracking for ammonia). This fuel flexibility can be attractive for a vessel owner. When combined with other waste heat recovery systems SOFCs can achieve electrical efficiencies 60% and higher.
Disadvantages: While the high operating temperature is an advantage in one respect, it also leads to accelerated degradation and corrosion of some system components. Also, higher quality (and often more expensive) heat-resistant materials are required to enable to reaction, so these fuel cells are often more expensive in comparison to PEM. SOFCs also take longer to start-up and are not as easy to cycle on and off.
A hydrogen internal combustion engine (HICE) differs from a fuel cell in that the hydrogen is explosively burned in the combustion chamber to produce mechanical energy as opposed to electrical energy. A HICE could be designed to run purely on hydrogen, or more commonly designed as dual-fuel engine where it can use hydrogen and some other hydrocarbon fuel like LNG or diesel.
Advantages: Hydrogen can be used as a fuel in an ICE over a wide range of fuel-air mixtures, even very lean mixtures. In a HICE the hydrogen does not need to be as pure as used in a fuel cell. Blending hydrogen with diesel can increase the efficiency of the internal combustion engine while reducing fuel consumption. Internal combustion engines are well established in the maritime industry and the maritime workforce is familiar with their operation.
Disadvantages: Hydrogen is difficult to ignite in a diesel engine that relies upon compression ignition (as opposed to spark plugs), because its autoignition temperature is so high; using a pilot fuel with a lower autoignition temperature (e.g., diesel) to initiate combustion can mitigate this challenge. Emissions from a HICE operating on pure hydrogen do not contain any carbon based pollutants, but it can produce oxides of nitrogen known as NOx (especially at high engine loads) which is a harmful pollutant.
Thanks for subscribing to The Liquid Grid! You’ll receive email updates when new articles are posted.